TCTC for Pediatric Infectious Disease
Children bear the heaviest burden of infectious illness. An island-wide or even international multi-institution collaboration is especially needed to accomplish any clinical trial in the field of pediatric infectious diseases. This is the grounds that we established the first Taiwan Pediatric Infectious Diseases Alliance (TPIDA) in 2009.
TPIDA, led by Professor Li-Min Huang of National Taiwan University Hospital, is a collaborative consortium of pediatric institutes in medical centers around the island. Since 2010, we together have already accomplished many joint researches regarding pediatric infectious diseases, and more studies about vaccine efficacy and effectiveness are on the way to establish Taiwan Pediatric Diseases Clinical Trial Consortium (TPDCTC).
- This consortium has wide-ranging experiences of collaboration with local/ international research institutes/pharmaceutical companies and CDC to examine new diagnostic test, therapeutic drugs, or vaccines. We have conducted various clinical trials in the past few years, including antiviral small molecule, seroprevalence of diseases in Taiwanese children, the efficacy and effectiveness of vaccines against vaccine preventable diseases, the immunogenicity and safety of unlisted vaccines, pre-registration trial for new medical devices and detection kit, and the surveillance of pathogens for acute gastroenteritis.
- Maintained the largest pediatric respiratory tract virus bank in Taiwan. Through the successful establishment of human airway epithelium cell culture (HAE), we could test the host-viral interaction and outcomes by using a more relevant ex vivo model.
- Demonstrated extraordinary ability to perform nationwide clinical trials for pediatric infectious diseases in Taiwan. Our recruitment capacity has increased to hundreds of young children and very young infants for recent sponsored clinical trials outperforming many competing sites in other countries.
- Holds a variety of educational symposia for researchers and clinicians to understand pediatric infectious diseases, especially in the field of viral infection.
International cooperation
- This alliance is entrusted by international pharmaceutical companies to conduct pre-market clinical trials of children's vaccines and early clinical trials of antiviral drugs, and also jointly publish research results after the trials.
- Respiratory fusion virus (RSV) vaccine. Assisted an US Company in executing the III phase clinical trial of the experimental RSVpreF vaccine program, targeting healthy infants and young children to evaluate the clinical efficacy and safety of the experimental RSVpreF vaccine.
- Alliance members also actively participate various international seminars related to children infectious diseases, and get the new knowledge from international pediatric infectious disease research. We also display research results to the international, attracting international research teams to propose cooperation plans and participate in global children's research.
- This alliance dispatches clinicians and clinical trial personnel to international early clinical trial institutions to study and receive long-term and short-term training. After returning from the training, clinicians also participated in the training course as a lecturer to cultivate more talents for early clinical trials of pediatrics in Taiwan.
Economic aspects
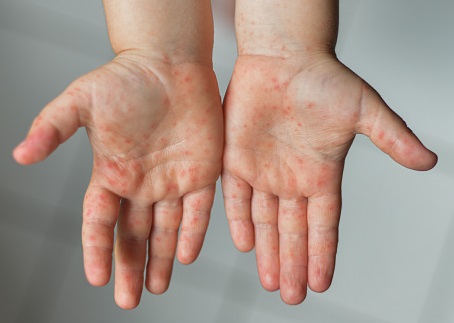
- Domestic enterovirus 71 (EV71) vaccine for children. Taiwan independently developed a vaccine for the first time and successfully completed Phase III multinational clinical trials! It has successfully applied for a drug license and will be launched in August this year. If it is successfully launched in the future, it will be bring great economic benefits.
- Domestic influenza vaccine. Assisted biotech company in completing the III phase of a domestic multi-center clinical trial to evaluate the immune production and safety of the quadrivalent inactivated influenza vaccine in healthy subjects aged 3 to less than 18 years old.
- COVID-19 Vaccine (MVC COVID-19 Vaccine). Assisted domestic biotech company in completing the II phase of domestic multi-center clinical trials to evaluate the safety, tolerability and immune production of the SARS-CoV-2 vaccine candidate MVC-COV1901 in adolescents.
- Continue to assist. domestic biotech company to complete and execute clinical trials related to COVID-19 vaccines. COVID-19 is expected to become a seasonal illness like most respiratory viruses. To prevent COVID-19, it could be need vaccinate every year in the future. High safety and broad protection will be the main development direction of the new COVID-19 in the future. If the clinical trial is successful, it will help the subsequent economic benefits will be considerable.

Social influence
- This alliance has been assisting two domestic biotech manufacturers to conduct clinical trials of children's vaccines for a long time. To strengthen Taiwan's independent production capacity for vaccines, and increase vaccine options for infants and children.
- During the COVID-19 epidemic, in addition to assisting Taiwan's domestic biotech manufacturers. in completing COVID-19 vaccine-related clinical trials, this year we also assisted. in executing the III phase clinical trials of the enterovirus 71 (EV71) vaccine program for children, and successfully applied for a drug license in April and was successfully launched in August this year.
- Since its establishment in 2012, the alliance has assisted a total of 27 clinical trial cases from domestic and foreign manufacturers, with the number of cases received reaching 2,300 cases, assisting in the successful launch of 8 vaccines and drugs, protecting people's lives and improving the health and well-being of all mankind.
- The COVID-19 virus is constantly mutating, threatening the health of the people. We will continue to assist domestic manufacturers in the development of new COVID-19 vaccines, and hope to protect the people health. The rate of COVID-19 infection and hospitalization increase among children. In this year, the Alliance has launched a number of clinical studies related to COVID-19.
Important notes of alliance
- Assist US company in executing the third phase clinical trial of the experimental RSVpreF vaccine program (targeting healthy infants and young children to evaluate the clinical efficacy and safety of the experimental RSVpreF vaccine; NCT04424316). The research results of this trial have been published in The New England Journal of Medicine in April 2023. The U.S. Food and Drug Administration (FDA) approved Pfizer's Respiratory Syndrome Virus (RSV) vaccine on August 21, 2023, for use in pregnant women in the second trimester of their third trimester to protect their babies.
-
- Assisted the phase III clinical trial of Taiwan domestic biotech company’s EV71 vaccine program. (A Phase III, multiple-center, double-blind, randomized, placebo-controlled study to evaluate the efficacy, immunogenicity, and safety of an adjuvanted inactivated Enterovirus 71 vaccine in healthy infants and children; NCT05099029). This research was published in the journal of Lancet. This is the first time Taiwan has independently completed the first to third phases of vaccine development. It also submits a New Drug Application (NDA) for Enterovirus A71 Vaccine to the Taiwan FDA in April 2023.
- Assisted the phase II clinical trial of Taiwan domestic biotech company’s COVID-19 vaccine. (Phase II, prospective, double-blinded, multi-center study to evaluate the safety, tolerability, and immunogenicity of the SARS-CoV-2 vaccine candidate MVC-COV1901 in adolescents; NCT04951388). This research was published in the journal of medRxiv.
- Assisted Taiwan domestic biotech companyAdimmune Corporation to complete relevant clinical trials of a influenza vaccine (for healthy subjects aged 3 to less than 18 years old, to evaluate the immune generation and safety of the quadrivalent inactivated influenza vaccine Phase 3 clinical trial; NCT04101435). This clinical data has been published in the international medical journal "Vaccine".
Children Hospital, National Taiwan University Hospital, President
Department of Pediatrics, National Cheng Kung University Hospital, President
Department of Pediatrics, National Cheng Kung University Hospital, Attending Physician
Department of Pediatrics, National Taiwan University Hospital, President
Department of Pediatrics, National Taiwan University Hospital, Attending Physician
Department of Pediatrics, National Taiwan University Hospital, Attending Physician
Department of Pediatrics, National Taiwan University Hospita,l Attending Physician
Department of Pediatrics, Mackay Memorial Hospital, Attending Physician
Department of Pediatrics, Mackay Memorial Hospital, President
Department of Pediatrics, Mackay Memorial Hospital, Attending Physician
China Medical University HospitalChina Medical University Childrens Hospital, Attending Physician
Section of Infectious Diseases , Hualien Buddhist Tzu Chi General Hospital, Attending Physician
Department of Pediatrics, Chang Gung Medical Foundation , Attending Physician
Department of Pediatrics, Chang Gung Medical Foundation , Attending Physician
Department of Pediatrics, Chang Gung Medical Foundation , Attending Physician
Department of Pediatrics, National Taiwan University Hospital Yunlin Branch , Attending Physician
National Health Research Institutes
Contact Information
c-IRB