Despite the advance in medical care, cardiovascular diseases (CVD's) are among the leading causes of death in the world. New diagnostic and therapeutic modalities have been vigorously developed to reduce the CV mortality and morbidity. To achieve the above goals, it is important to conduct multicenter trials in a more efficient way by a clinical trial consortium. The major tasks of TCTC-CVD include:
1. To establish and maintain a clinical trial consortium for CVD’s in Taiwan, which can conduct standardized multi-center clinical trials for CVD’s, promote international academic communication and cooperation, hold international seminars, and provide international clinical trial information and the platform to share the resources and information of this consortium.
2. Through multi-center clinical trials to define the best diagnosis and treatment strategies for cardiovascular diseases.
3. To collect demographic data, clinical information, laboratory data, biological samples, and establish an electronic database, which can be used for basic research, clinical research, and a service platform for the biomedical industry.
I. International cooperation:
1. Novolimus Eluting Coronary Bioadaptor System
- Class III new medical device/material
- Indications: patients with symptomatic ischemic heart disease with discrete primary lesions in native coronary arteries.
The trial "Comparison of long-term vascular adaptability changes and efficacy of new vascular adaptable coated stents and traditional coated stents in native coronary artery disease" integrated and implemented by this consortium has been completed. This study used CT-coronary angiography to compare the new vascular adaptable drug-coated stent and the traditional drug-coated stent to change the geometric structure of the coronary arteries, including curvature, diameter and length. The results were better than those of the traditional drug-coated stent. This trial supports the FDA's approval of this class-III new medical device for marketing in March 2011.
2. Semaglutide
- Drug phase III
- Indications: diabetes, obesity.
The trial " Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT)」" integrated and implemented by this consortium has been completed, enrolling 17,500 cases worldwide. There were 157 cases from Taiwan (17 more than expected, ranking the second place in case enrollment efficiency in the world). This trial intended to evaluate whether semaglutide could reduce CV events in patients who were overweight or obese and had previous CVD’s (including myocardial infarction, stroke and peripheral arterial disease). On August 8, 2023, the Denmark company announced the important results SELECT trial: Semaglutide can reduce the incidence of major cardiovascular events by 20%. The detailed results will be announced at the American Heart Association Congress in November this year, published as landmark trial. This is the first time over the world to prove that weight-loss drugs can not only reduce weight, but also reduce cardiovascular disease and mortality, which would greatly change the medical community's view of weight-loss drugs. The Taiwanese performance for this trial was very good. The leader of this consortium, Dr. Wu, also participated in its Global Expert Panel (GEP). Although the Asian headquarters of the Denmark company was located in Mainland China, Mainland was unable to conduct such clinical trials due to relevant laws and regulations. Therefore, due to Taiwanese excellent performance in clinical trials, the Denmark company recommended TCTC-CVD to lead this clinical trial in Taiwan and to collect a larger amount of data for Chinese people. This case is of considerable help for the cultivation of relevant talents and international cooperation, and is one of the sparks of this consortium.


II. Economic impact:
1. Alzheimer's disease immunomagnetic reduction detection reagent
- Class III new medical device/material
- Indications: Early diagnosis of Alzheimer's disease
In the past, Alzheimer's disease was usually diagnosed with MRI or spinal fluid testing. The Institute of Optoelectronic Engineering of National Taiwan Normal University collaborated with our consortium to design domestic multicenter human trials and develop "immune magnetic reduction testing technology", which can be done with a tube of 6cc blood, to detect early-stage Alzheimer's disease patients. These trials lead to TFDA approval of "Alzheimer's disease immunomagnetic reduction detection reagent”, with marketing authorization from Taiwan's Ministry of Health and Welfare (third-level new medical material) in 2020.12.
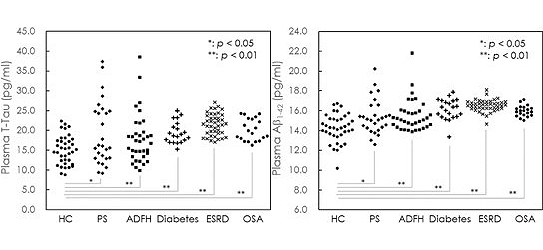
 Sci Rep 2022 Jan 24; 12(1): 1192
Sci Rep 2022 Jan 24; 12(1): 1192
2. Application of Multi-channel ECG Signal Measurement and Analysis in Cardiovascular Diseases
- Class II new medical device/material
- Indications: Cardiovascular diseases
“Application of Multi-channel ECG Signal Measurement and Analysis in Cardiovascular Diseases” created by the leader of this consortium has obtained the support from the National Seedling Program of Taiwan MOST. This instrument is the first one in the world to use resting ECG to diagnose stable coronary artery disease. It is planned to help establish a biotech startup company, to create job opportunities and promote the development of related biotechnology industries.
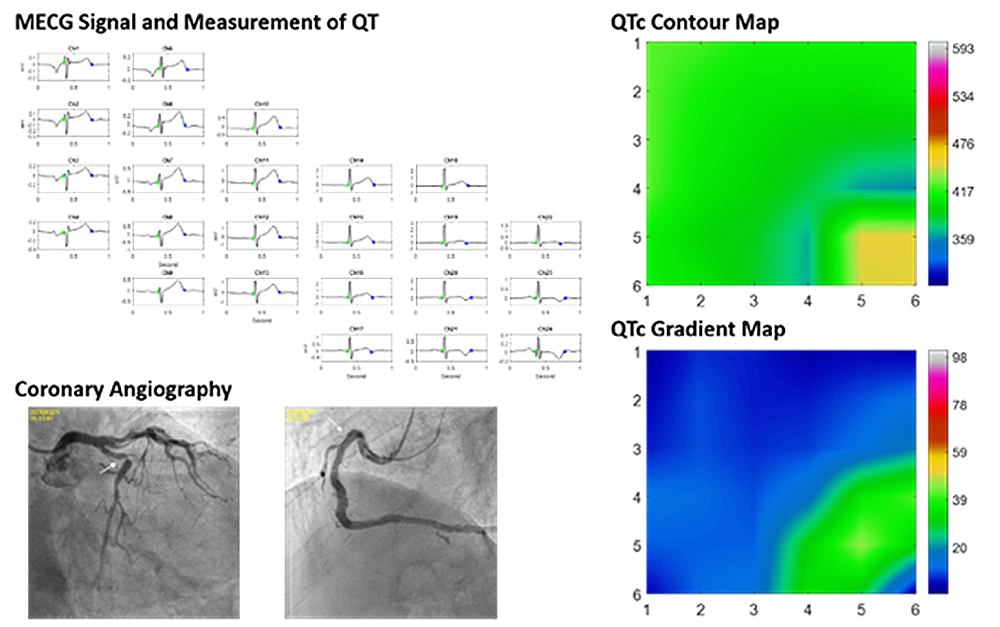
3. New formulation of activated bamboo charcoal (CharXenPlus, ProtectRenal)
- Drug phase II
- Indications: Chronic kidney disease
The consortium has designed a human trial to explore the use of a locally developed new formulation of activated bamboo charcoal/probiotics to adsorb nephrotoxins in the intestines to accelerate their excretion from the body, as a new method for preventing cardiovascular complications in patients with chronic kidney disease. This new formulation of activated bamboo charcoal is a patented product that could compete with Japanese drugs.
III. Social impact
TCTC-CVD has conducted more than 100 clinical trials, and From 2013 the "Taiwan Dyslipidemia Primary and Secondary Prevention Registration Cohort Study" has 14,346 cases have been enrolled. The information collected from this cohort has been used to investigated the current status of domestic patients with hypertension and hyperlipidemia, which can provide evidence for designing medical policy. The results have also become an important reference for Taiwan to formulate blood lipid treatment guidelines. On August 1, 2013, Taiwan National Health Insurance Administration (NHIA) revised the reimbursement standards for lipid-lowering drugs based on the data of this study; and the Taiwan Society of Lipid and Arteriosclerosis also revised the Taiwanese blood lipid treatment guidelines for ACS patients in 2017, and the THIA updated again on February 1, 2019, according to this treatment guideline, the lipid-lowering drug reimbursement standards for patients with ACS and CAD who have undergone interventional surgery. It is hoped that the number of cardiovascular events in Taiwan will be greatly reduced in the future, and related medical expenditures will be reduced.
IV. Important notes of the consortium
- Publications of clinical trials for registration:
- Tang SC, et al. Plasma β-amyloids and Tau Proteins in Patients with Vascular Cognitive Impairment. Neuromol Med. 2018;20:1-6.
- Lue LF, et al. Age-dependent relationship between plasma Aβ40 and Aβ42 and total tau levels in cognitively normal subjects. Front. Aging Neurosci., 2019;11:1-8.
- Pai MC, et al. Evidence of plasma biomarkers indicating high risk of dementia in cognitively normal subjects. Sci Rep 2022 Jan 24; 12(1): 1192.
- Records of award:
- In 2016, Kao, H.L., Huang, C.C. (both from NTUH) and Xiao, H.M. from the Department of Mechanical Engineering of National Taiwan University were awarded with the 13th Academic Research Innovation Award for their "non-invasive imaging technology for rapid assessment of stroke and cardiovascular disease risk". In 2021, the new startup company "Boxiang Medical Technology" cooperated with this project was selected as one of the Top 10 Startups in Taiwan (Top 10 Startups in Taiwan 2021) by the American Startup City magazine, further gaining international attention. In the same year, the company won the championship in the X-Pitch 2021 International Extreme Sports Competition. In 2022, it was listed in the 2021 Social Responsibility and Sustainability Report of National Taiwan University. In the same year, the researchers were interviewed and reported by many domestic and foreign media, including US News. In 2024, the company won the "Enterprise Startup Award-Innovative Medical Materials and Diagnostic Technology Category" of the 21st National Innovation Award, demonstrating the company's development potential.
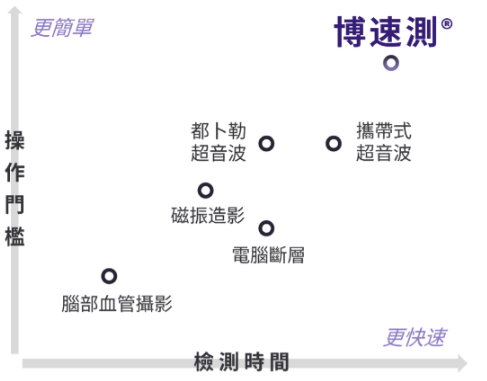
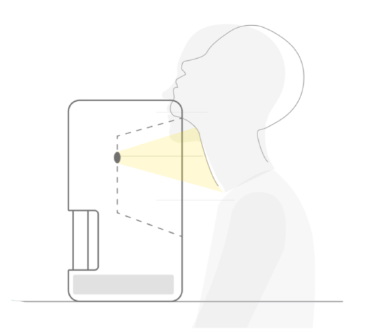
- Wu, C.C. Real-time cardiovascular function assessment system with surface multichannel ECG signal analysis: 2018 Future Science and Technology Breakthrough Award of the Ministry of Science and Technology (2018/12/15) In 2025, we obtained the "Production and Marketing Plan of Mobile 30-channel Electrocardiogram Testing System" from the Ministry of Economic Affairs, R.O.C.’ Value Creation Program (signed on February 27, 2025), and established a new company "Chung-Mei Biopharma Co., Ltd."
- Chen MJ, Wu CC, Lee YJ. Developing probiotic-assisted therapy for chronic kidney disease: The 19th (2022) National Innovation Award (2022/12/4)
- Papers for treatment guidelines:
- Li YH, et al. 2017 Taiwan lipid guidelines for high-risk patients. J Formos Med Assoc. 2017 Apr;116:217-248.
- Li YH, et al. A performance guide for major risk factors control in patients with atherosclerotic cardiovascular disease in Taiwan. J Formos Med Assoc. 2020 Mar;119(3):674-684.
- Hsiao YC, et al. A risk stratification model modified from the U.S. guideline could be applied in an Asian population with or without ASCVD. Biomed J. 2023 Aug 12:100653.







