International Collaboration:
(1)Our consortium members all have experience in conducting international clinical trials. Taking the years 2020 to 2023 as an example, we have conducted over 30 new clinical trials related to esophageal and GEJ tumors. Our collaboration partners include globally renowned pharmaceutical companies from the United States, the United Kingdom, Japan, and other countries.
(2)Our consortium has participated in numerous multinational clinical trials, resulting in publications in international journals. The following are recent papers published within the last two years, serving as foundational data for drug approval prior to market entry.
A.The Lancet Oncology (2023 May;24(5):483-495)/ NCT03783442
B.Clinical Cancer Research (2023 Oct 2;29(19):3882-3891)/ NCT03505320
C.Journal of Clinical Oncology (2022 Sep 10;40(26):3065-3076)/ NCT03430843
D.Cancer (2022 Mar 1;128(5):995-1003)/ NCT03019588
(3)Actively collaborate with global pharmaceutical companies to assist domestic and international companies in accelerating the development timeline of biomedical products and marketing approval, enhancing Taiwan's visibility and competitiveness in the treatment of esophageal and GEJ cancers on the international stage.
Economic Benefit:
A successful late-phase clinical trial is the final milestone to advance a novel drug into the market. In order to provide patients with more and better treatment options, our consortium assists both domestic and international pharmaceutical companies in conducting clinical trials to obtain marketing approval of drugs for treating esophageal or GEJ cancers. The following are examples of drugs for which relative indications have been obtained due to positive trial outcomes.
|
FDA-Approved Drugs |
Clinical Trials |
| (1) |
Nivolumab |
NCT02569242, NCT02743494, NCT03143153 |
| (2) |
Pembrolizumab |
NCT03189719 |
| (3) |
Tislelizumab |
NCT03430843 |
Social Impact:
The global medical industry, including pharmaceutical researchers and hospital physicians, aims to provide patients with better treatment options through continuous innovation in drug development. Although investigational products in clinical trial stage are not part of standard treatment and may face patient resistance. However, countries worldwide have established rigorous regulations for clinical trials. Each trial protocol must undergo scrutiny by health authorities and hospital management department to minimize risks to patients participating in clinical trials. Regulatory authorities also inspect the conduct of clinical trials and review their outcomes before approving a drug for market entry, ensuring that the benefits of the drug outweigh its risks.
Beyond assisting new drug approvals, clinical trials also offer additional benefits to society and the public, such as:
(1)Patients have the opportunity to access the latest therapies or medications globally by participating in clinical trials, with all expenses covered by the trial sponsor. However, this involvement may pose risks, including potential adverse events. According to regulations, patients have the freedom to decide whether or not to participate in clinical trials.
(2)Taiwan boasts one of the world's best universal healthcare systems, wherein patients benefit from significantly reduced medication costs provided that the drugs meet the reimbursement criteria of the National Health Insurance (NHI). Among the drugs currently facilitated to market by our consortium, nivolumab has met the reimbursement criteria of the NHI as outlined below.
A.In April 2020, approval for adult patients with recurrent or metastatic esophageal squamous cell carcinoma, including GEJ cancer, who had previously failed second-line chemotherapy.
B.In April 2024, nivolumab 120mg was approved for patients with unresectable advanced, recurrent or metastatic esophageal squamous cell carcinoma after prior fluoropyrimidine- and platinum-based chemotherapy.
C.In April 2024, nivolumab 120mg was approved for combination with fluoropyrimidine- (5-FUor capecitabine) and oxaliplatin chemotherapy. It is indicated for the first-line treatment of adult patients with HER2-negative advanced or metastatic gastric including GEJ cancer.
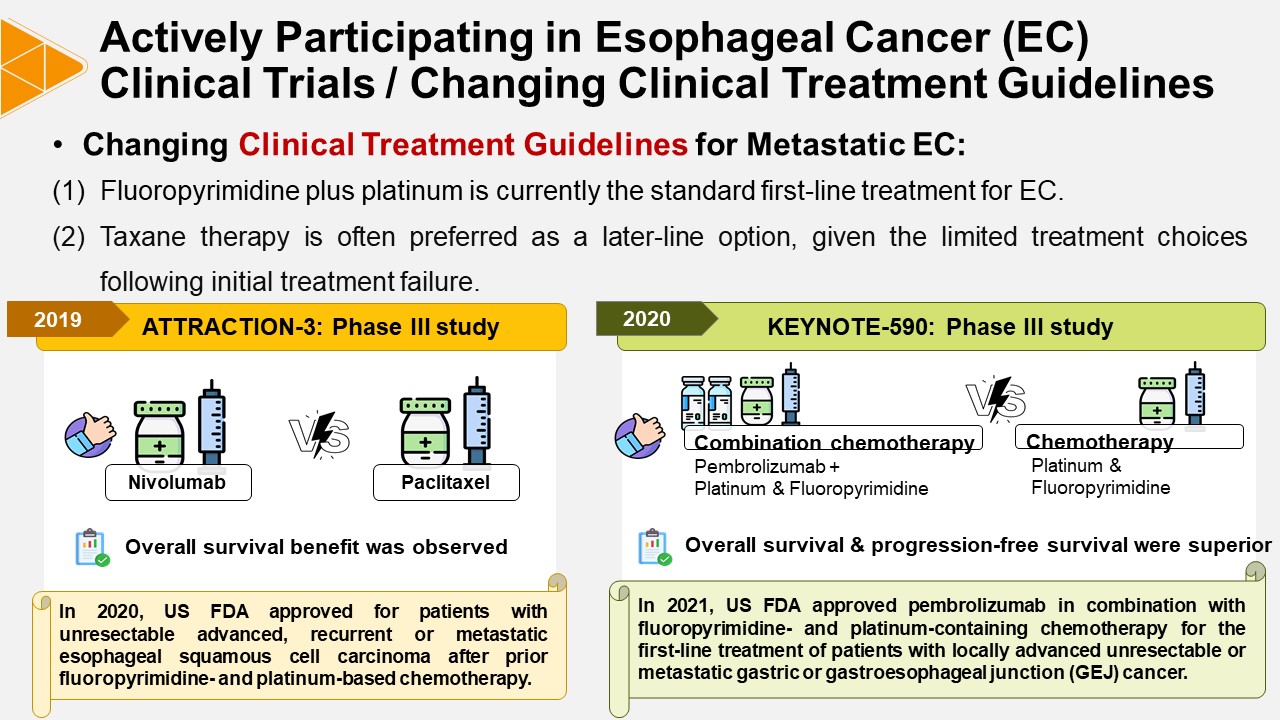
Milestone:
The international pivotal clinical trials on esophageal and GEJ cancers in which our institute participated have obtained multiple FDA indications approvals. Among these, the two pivotal trials with the earliest initiation and longest follow-up periods were conducted between 2015-2021 and 2015-2018 respectively: "A Multicenter, Randomized, Open-label Study in Patients with esophageal Cancer refractory or intolerant to Combination Therapy with Fluoropyrimidine and Platinum-based Drugs (ATTRACTION-3) (NCT02569242)" and "ONO-4538 Phase III Study: A Multicenter, Double-Blind, Randomized Study in Patients with Unresectable Advanced or Recurrent Gastric Cancer (NCT02267343)". Both clinical trials resulted in FDA approvals for investigational products in 2020 and 2021, applicable to esophageal squamous cell carcinoma, gastric cancer, GEJ cancer, and esophageal adenocarcinoma, thereby hanging clinical treatment guidelines.