1. Heart failure is a devastating condition, leading to a high mortality rate and a troubling economic burden. Over the past 40 years, the concept and treatment for this disease entity have continued evolving. Conceptually, heart failure has been transformed from a volume disease related to renal hemostasis, a hemodynamic disease, to a neurohormonal disease. Along with these changes in our basic understanding of heart failure, new treatments have improved the survival of those who suffer gravely from heart failure. Nevertheless, the mortality rate for a patient with heart failure is still around 10% for the first year with the best contemporary treatment. On top of it, rehospitalization of heart failure occupying a big sector of cardiac inpatient care occurs in 30% of these patients.
2. In order to conduct high-quality heart failure clinical trials and improve the quality of care for heart failure patients, we have set up the Taiwan Clinical Trial Consortium for Heart Failure.
3. Our past records in the field of heart failure management have brought many international clinical trials into Taipei Veterans General Hospital. With our own path in the clinical trials of heart failure, we witness the changes of this fast-moving field. Furthermore, we would like to shed our lights on other centers that have an aim to advance heart failure knowledge.
Because CVD's are a group of disorders of the heart and blood vessels, including: coronary heart disease, cerebrovascular disease, peripheral arterial disease, valvular heart disease, congenital heart disease, deep vein thrombosis and pulmonary embolism, arrhythmia, and heart failure, etc., we have divided our PI and Co-PI's from 14 medical centers into 12 disease-specific branches according to their sub-specialties in CVD's.
Each PI or Co-PI is in charge or co-charge of one branch to integrate and run the clinical trials in a more efficient way, respectively. Each branch could also cooperate with other branches to run one trial if necessarily, to enroll the patients quicker and save the man-power and expense. The major tasks of the branch PI and Co-PI are not only to integrate and help the existing trials run by each PI invited by the Taiwanese CRO and to attract the international medical or pharmaceutical companies to run their clinical trials in Taiwan, but also and most importantly, to help the domestic medical or pharmaceutical companies to run their clinical trials and research/development of new products. The 12 branches include:
- Arrhythmia and sudden cardiac death
- Cardiovascular biosignature and cardiac oncologyy
- Surgery for CVD’s and cardiorenal syndrome
- Diabetes and cardiovascular disease
- Heart failure, valvular heart disease and its intervention
- Hypertension
- Ischemic heart disease and its intervention
- Lipid, atherosclerosis, and vascular dementia
- Peripheral vascular diseases
- Pulmonary artery hypertension and resuscitation/hypothermia
- Thromboembolism, antiplatelets, and anticoagulants
- Cardiovascular image and miscellaneous
Global achievement and industry collaboration:
1. Dr Chern-En Chiang, the chair of Taiwan Clinical Trial Consortium for Heart Failure, has been the national lead investigator of multiple international clinical trials for heart failure diagnostics and therapeutics, shown below. Our model of success includes:
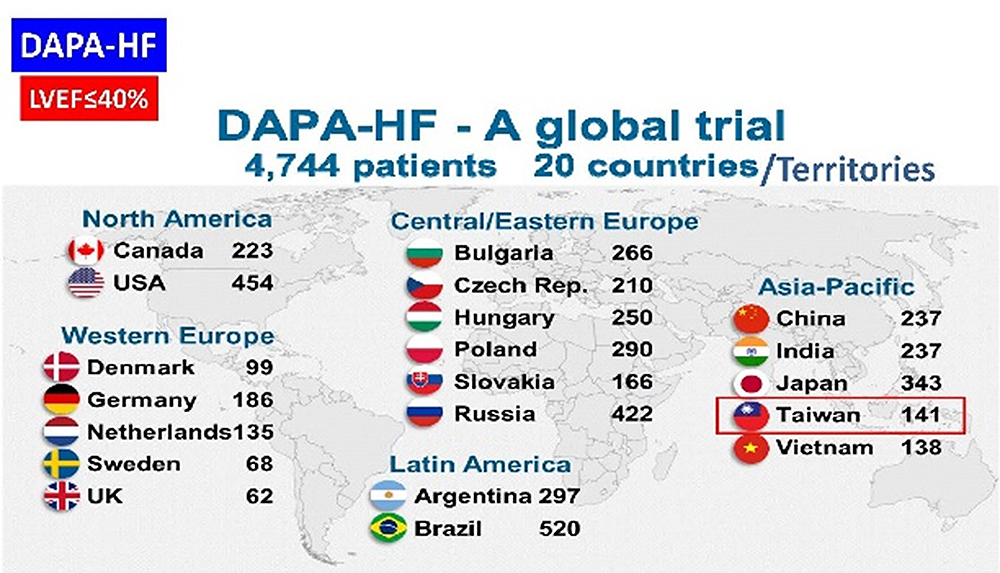
- 1. Protocol no.:D1699C00001 [DAPA-HF]
- Phase:III
Locations: Taiwan、China、India、Japan、Vietnam、Bulgaria、Czech、Denmark、Germany、Hungary、Netherlands、Poland、Russia、Slovakia、Sweden、UK、Argentina、Brazil、Canada、US
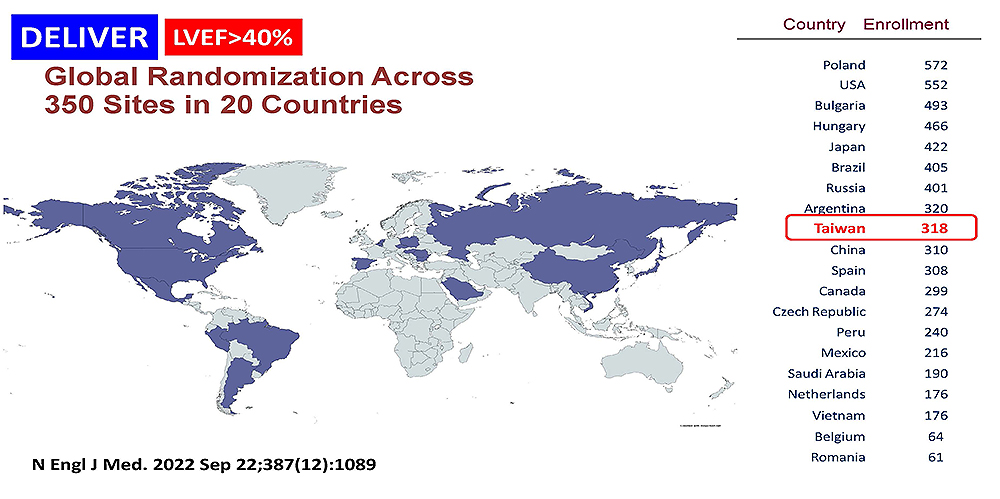
- 2. Protocol no.:D169CC00001 [DELIVER]
- Phase:III
Locations: Taiwan、China、Japan、SaudiArabia、Vietnam、Bulgaria、Czech、France、Hungary、Netherlands、Belgium、Poland、Romania、Russia、Spain、Argentina、Brazil、Mexico、Peru、Canada、US
- 3. Protocol no.:MK1242-001 [VICTORIA]
- Phase:III
Locations: Taiwan、Austria、Belgium、Czech Republic、Denmark、Finland、France、Germany、Hungary、Italy、Netherlands、Norway、Poland、Spain、Sweden、Switzerland、UK/Ireland、Israel、Russia、South Africa、Turkey、Ukraine、Argentina、Brazil、Chile 、Colombia、Mexico、Puerto Rico、Australia/New Zealand、China、Japan、South Korea、Malaysia、Philippines、Singapore、Hong Kong、Canada、United States
- 4. Protocol no.:D6402C00001 [MIRACLE]
- Phase:II
Locations: Taiwan、Lithuania、Poland、Russia、Slovakia、Spain、Sweden、Ukraine、Japan、Canada、United States、South Korea、Thailand、Belgium、Bulgaria、Czech Republic、Denmark、Germany、Hungary、Italy
- 5. Protocol no:BAY20103 [FINEARTS-HF]
- Phase:III
Locations: Taiwan、 Argentina、Australia、Austria、United Kingdom、Netherlands、New Zealand、Poland、Portugal、Slovakia、South Africa、Spain、Ukraine、United States、Mexico、Belgium、Brazil、Bulgaria、Canada、China、Colombia、Czech Republic、Denmark、Estonia、Finland、France、Germany、Greece、Hong Kong、Hungary、India、Israel、Italy、Japan、South Korea、Latvia、Lithuania、Malaysia、Sweden、Norway、Turkey、Romania、Russia、Singapore
- 6. Protocol no:I8F-MC-GPID [SUMMIT]
- Phase:III
Locations: Taiwan、Argentina、Brazil、China、India、Israel、Mexico、Russia、United States
- 7. Protocol no:D6580C00010 [ENDEAVOR]
- Phase:IIb/III
Locations: Taiwan(only Asia country)、 Argentina、Brazil、Bulgaria、Canada、Czech Republic、Denmark、France、Hungary、Japan、Poland、Russia、Slovakia、Sweden、United States
- 8. Protocol no:MK1242-035 [VICTOR]
- Phase:III
Locations: Taiwan、Canada、United States、Brazil、Chile、Colombia、Mexico、Peru、Italy、Poland、Russia、South Africa、Spain、Sweden、Turkey、Puerto Rico、Austria、Czech Republic、Denmark、France、Germany、Greece、Hungary、Israel、United Kingdom、Ukraine 、Australia、China、Hong Kong、Malaysia、New Zealand、Singapore、South Korea
- 9. Protocol no:EX6018-4915 [HERMES]
- Phase:III
Locations: Taiwan、Romania、Lithuania、Republic of North Macedonia、Malaysia、Mexico、New Zealand、Norway、Poland、Portugal、Serbia、Singapore、Slovakia、Slovenia、South Africa、South Korea、Spain、Thailand、Turkey、United Kingdom、United States、Argentina、Australia 、Austria、Belgium、Bosnia、Brazil、Bulgaria、Canada、China、Colombia、Croatia、Czech Republic、Denmark、Estonia、Finland、France、Germany、Greece、Hungary、India、Ireland、Israel、Italy、Japan、Latvia
2. Dr Chern-En Chiang, the chair of Taiwan Clinical Trial Consortium for Heart Failure, has been sitting on the steering committee of multiple international clinical trials for heart failure diagnostics and therapeutics.
- 1. Protocol no.:D1699C00001 [DAPA-HF]
- Phase:III
Locations: Taiwan、China、India、Japan、Vietnam、Bulgaria、Czech、Denmark、Germany、Hungary、Netherlands、Poland、Russia、Slovakia、Sweden、UK、Argentina、Brazil、Canada、US
- 2. Protocol no.:D169CC00001 [DELIVER]
- Phase:III
Locations: Taiwan、China、Japan、SaudiArabia、Vietnam、Bulgaria、Czech、France、Hungary、Netherlands、Belgium、Poland、Romania、Russia、Spain、Argentina、Brazil、Mexico、Peru、Canada、US
- 3. Protocol no.:MK1242-001 [VICTORIA]
- Phase:III
Locations: Taiwan、Austria、Belgium、Czech Republic、Denmark、Finland、France、Germany、Hungary、Italy、Netherlands、Norway、Poland、Spain、Sweden、Switzerland、UK/Ireland、Israel、Russia、South Africa、Turkey、Ukraine、Argentina、Brazil、Chile 、Colombia、Mexico、Puerto Rico、Australia/New Zealand、China、Japan、South Korea、Malaysia、Philippines、Singapore、Hong Kong、Canada、United States
- 4. Protocol no.:EX6018-4915 [HERMES]
- Phase:III
Locations: Taiwan、Romania、Lithuania、Republic of North Macedonia、Malaysia、Mexico、New Zealand、Norway、Poland、Portugal、Serbia、Singapore、Slovakia、Slovenia、South Africa、South Korea、Spain、Thailand、Turkey、United Kingdom、United States、Argentina、Australia 、Austria、Belgium、Bosnia、Brazil、Bulgaria、Canada、China、Colombia、Croatia、Czech Republic、Denmark、Estonia、Finland、France、Germany、Greece、Hungary、India、Ireland、Israel、Italy、Japan、Latvia

II. Economic aspect:
- 1. The DAPA-HF trial has been a great success. The US FDA approved dapagliflozin for adults with heart failure with reduced ejection fraction to reduce the risk of cardiovascular death and hospitalization for heart failure in May 2020. Following that, the Taiwan FDA gave dapagliflozin the same treatment indication in August 2021.
- The VICTORIA trial had made a breakthrough in heart failure management. The US FDA and the Taiwan FDA approved vericiguat for adults with symptomatic chronic heart failure and ejection fraction less than 45% to reduce the risk of cardiovascular death and heart failure hospitalization in January and July 2021.
- The DELIVER trial has been groundbreaking. The total budget for Taiwan was about TWD 300,000,000. The US FDA has approved an expanded label for dapagliflozin to include an indication to reduce the risk of cardiovascular death, hospitalization for heart failure, and urgent heart failure in adults. The Taiwan FDA has approved the same expanded label in August 2023.
III. Social impact:
Because of clinical trials like the DAPA-HF, VICTORIA, and DELIVER trials conducted through the network of Taiwan Clinical Trial Consortium for Heart Failure, these drugs receive approval for treatment in heart failure. Consistently, these new treatments provide a further 10% reduction in the mortality rate. This shows that our collaboration achieves our goal to improve care standard in heart failure. We are delighted to see patients with heart failure can now live longer and better.
IV. Milestone:
- 1. Key publications of clinical studies supporting regulatory approval
- (1) Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, Jhund PS, Belohlavek J, Chiang CE, Borleffs CJW, Comin-Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer-Gamba MA, Al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O'Meara E, Saraiva JFK, Tereshchenko SN, Thierer J, Vaduganathan M, Vardeny O, Verma S, Pham VN, Wilderäng U, Zaozerska N, Bachus E, Lindholm D, Petersson M, Langkilde AM; DELIVER Trial Committees and Investigators. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2022 Sep 22;387(12):1089-1098. doi: 10.1056/NEJMoa2206286.
- (2) McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019 Nov 21;381(21):1995-2008.
- (3) Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett J, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Vinh PN, Schou M, Tereshchenko S, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD, Johanson P, Greasley PJ, Boulton D, Bengtsson O, Jhund PS, McMurray JJV. Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes. JAMA. 2020 Apr 14;323(14):1353-1368.
- Vardeny O, Fang JC, Desai AS, Jhund PS, Claggett B, Vaduganathan M, de Boer RA, Hernandez AF, Lam CSP, Inzucchi SE, Martinez FA, Kosiborod MN, DeMets D, O'Meara E, Zieroth S, Comin-Colet J, Drozdz J, Chiang CE, Kitakaze M, Petersson M, Lindholm D, Langkilde AM, McMurray JJV, Solomon SD. Dapagliflozin in heart failure with improved ejection fraction: a prespecified analysis of the DELIVER trial. Nat Med. 2022 Dec;28(12):2504-2511. doi: 10.1038/s41591-022-02102-9.
- Butt JH, Dewan P, Merkely B, Belohlávek J, Drożdż J, Kitakaze M, Inzucchi SE, Kosiborod MN, Martinez FA, Tereshchenko S, Ponikowski P, Bengtsson O, Lindholm D, Langkilde AM, Schou M, Sjöstrand M, Solomon SD, Sabatine MS, Chiang CE, Docherty KF, Jhund PS, Køber L, McMurray JJV. Efficacy and Safety of Dapagliflozin According to Frailty in Heart Failure With Reduced Ejection Fraction : A Post Hoc Analysis of the DAPA-HF Trial. Ann Intern Med. 2022 Jun;175(6):820-830. doi: 10.7326/M21-4776.
- Butt JH, Jhund PS, Belohlávek J, de Boer RA, Chiang CE, Desai AS, Drożdż J, Hernandez AF, Inzucchi SE, Katova T, Kitakaze M, Kosiborod MN, Lam CSP, Maria Langkilde A, Lindholm D, Bachus E, Martinez F, Merkely B, Petersson M, Saraiva JFK, Shah SJ, Vaduganathan M, Vardeny O, Wilderäng U, Claggett BL, Solomon SD, McMurray JJV. Efficacy and Safety of Dapagliflozin According to Frailty in Patients With Heart Failure: A Prespecified Analysis of the DELIVER Trial. Circulation. 2022 Oct 18;146(16):1210-1224. doi: 10.1161/CIRCULATIONAHA.122.061754.
- Jhund PS, Ponikowski P, Docherty KF, Gasparyan SB, Böhm M, Chiang CE, Desai AS, Howlett J, Kitakaze M, Petrie MC, Verma S, Bengtsson O, Langkilde AM, Sjöstrand M, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Sabatine MS, Solomon SD, McMurray JJV. Dapagliflozin and Recurrent Heart Failure Hospitalizations in Heart Failure With Reduced Ejection Fraction: An Analysis of DAPA-HF. Circulation. 2021 May 18;143(20):1962-1972. doi: 10.1161/CIRCULATIONAHA.121.053659.
- Martinez FA, Serenelli M, Nicolau JC, Petrie MC, Chiang CE, Tereshchenko S, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Ponikowski P, Sabatine MS, DeMets DL, Dutkiewicz-Piasecka M, Bengtsson O, Sjöstrand M, Langkilde AM, Jhund PS, McMurray JJV. Efficacy and Safety of Dapagliflozin in Heart Failure With Reduced Ejection Fraction According to Age: Insights From DAPA-HF. Circulation. 2020 Jan 14;141(2):100-111. doi: 10.1161/CIRCULATIONAHA.119.044133.
- 2. Reward
- Outstanding performance award in recognition of the excellence in competing the VICTORIA trial was presented to Taipei Veterans General Hospital (Merck Sharp & Dohme; 2016).

- (2) Outstanding achievement award in recognition of performance and contributions in clinical trial leadership was presented to Taipei Veterans General Hospital (Eli Lily; 2023).

- 3. Clinical practice guidelines
Because of our leadership in heart failure management in Taiwan, our consortium leader has long been the national lead investigator and the member of steering committee of multiple international clinical trials for heart failure diagnosis and treatment. Apart from that, our consortium leader has been elected as the chair of guidelines committee of Taiwan Society of Cardiology, being in charge of setting up treatment standards for heart failure.
- 2018 consensus of the Taiwan Society of Cardiology and the Diabetes Association of Republic of China (Taiwan) on the pharmacological management of patients with type 2 diabetes and cardiovascular diseases. J Chin Med Assoc. 2018 Mar;81(3):189-222.
- 2020 Consensus of Taiwan Society of Cardiology on the pharmacological management of patients with type 2 diabetes and cardiovascular diseases. J Chin Med Assoc. 2020 Jul;83(7):587-621.
- 2021 Consensus Pathway of the Taiwan Society of Cardiology on Novel Therapy for Type 2 Diabetes. JACC Asia. 2021 Aug 28;1(2):129-146.
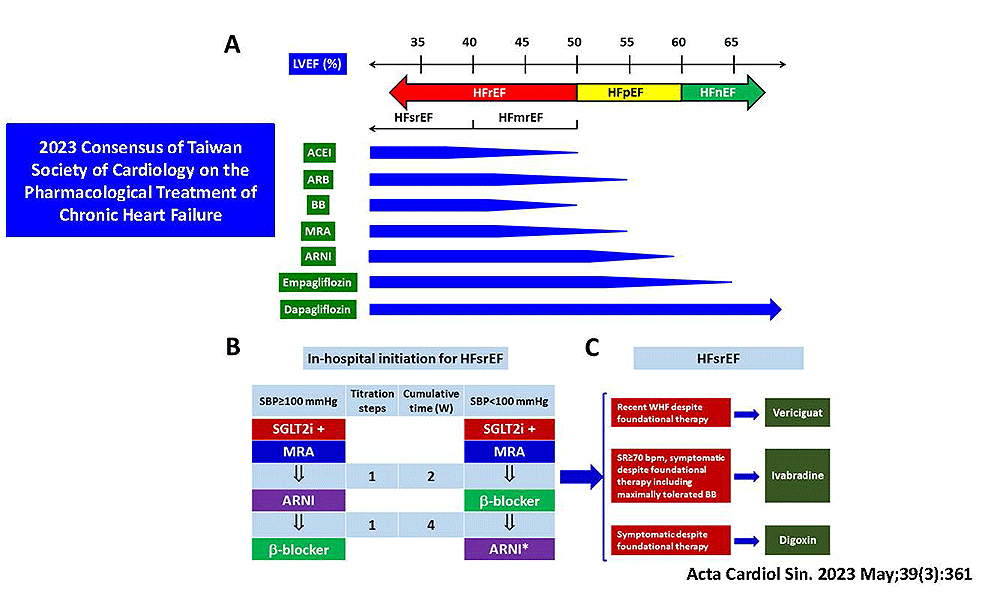
- 2023 Consensus of Taiwan Society of Cardiology on the Pharmacological Treatment of Chronic Heart Failure. Acta Cardiol Sin. 2023 May;39(3):361-390.