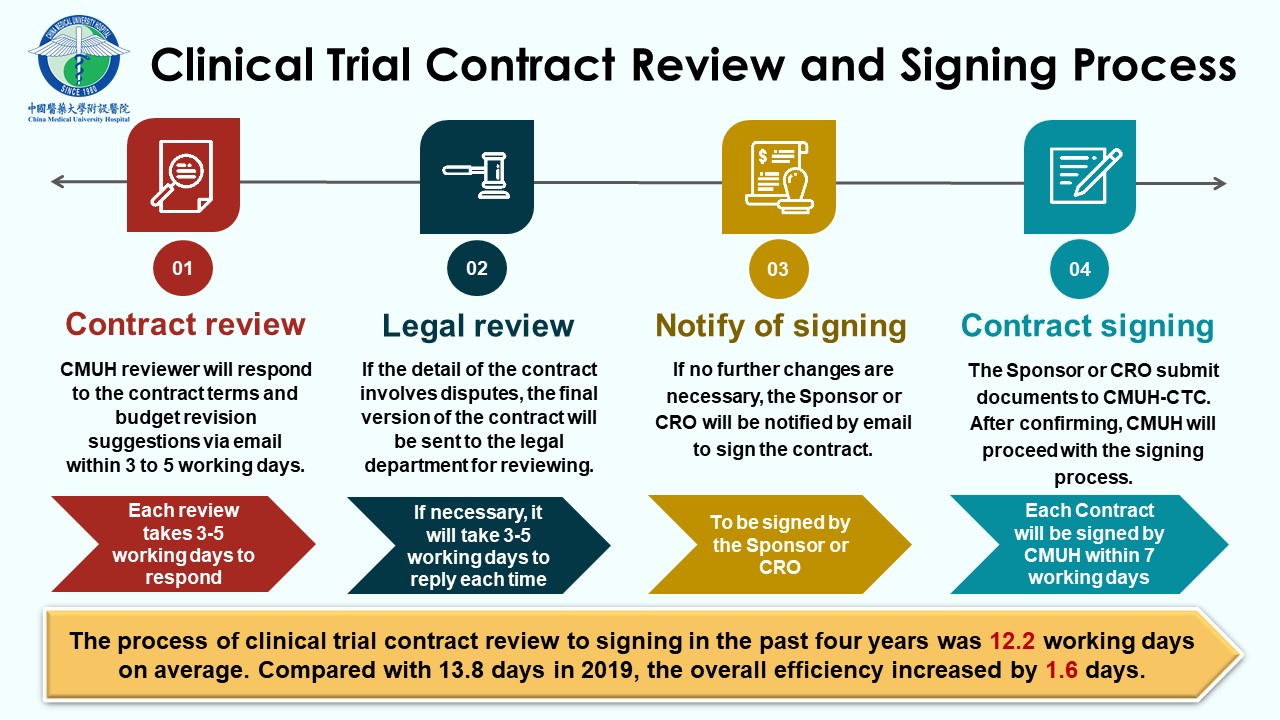
In 2010, our institution established the Clinical Trial Center (CTC) to provide a comprehensive service for conducting clinical trials, which are divided into five departments with functional and mutual support for each other, including the administration team, the in-house SMO team, the training and education team, the Academic Research Organization (ARO) team, and the DryLab team. Administration team with a primary mission to provide clinical trial integrated services, management, and implementation. To speed up the initiation of the clinical trial, CTC accepts English contracts. The contracts can be submitted for review in parallel with the Research Ethics Committee and the Food and Drug Administration of the Ministry of Health and Welfare, facilitating the prompt initiation of trials. Dedicated clinical research nurses from CTC provide all necessary services required for clinical trial enrollment, assist in the coordination of trial procedures, ensure seamless operation, and offer participants the most immediate, comprehensive, and professional care. In addition, the training and education team regularly organizes domestic and international clinical trial-related activities to improve the abilities and knowledge required in trial execution. Invite experts from industry, government, and academia to provide professional training courses in person and online to continuously acquire knowledge and improve Taiwan’s competitiveness.
CTC set up the nation‘s first Academic Research Organization, which offers "One-stop Comprehensive Services". Compared to commercial CROs, the ARO is more efficient and cost-effective. ARO provides a wide range of services that cover clinical trial consultation, protocol design, TFDA/IRB document submission, clinical trial project management and monitoring, data management and analysis, and clinical study report writing. ARO has extensive experience in conducting clinical trials, including multinational multicenter clinical trials such as “ATACH-II” and “EXTEND” studies. Its remarkable performance has been recognized on international stages, by CNN in the United States and the European Stroke Organization. Furthermore, the outcomes of these clinical trials have been published in high-ranking SCI journals such as “The Lancet” and “The New England Journal of Medicine”.
The DryLab team at CTC provides clinical trial data management and related statistical analysis services, such as sample size calculation, randomization, and statistical analysis reports. We also analyze big data, such as that from the Health and Welfare Data Science Center and the Taiwan Stroke Registry, to conduct real-world studies and generate real-world evidence. This kind of study assists in the conceptualization and execution of investigator-initiated trials or serves as supplementary evidence for post-marketing studies of drug efficacy and safety. Up to now, more than 400 SCI papers related to marketed drugs have been successfully published. Some dosing recommendations and warnings that differ from the existing package insert were indicated in those studies.
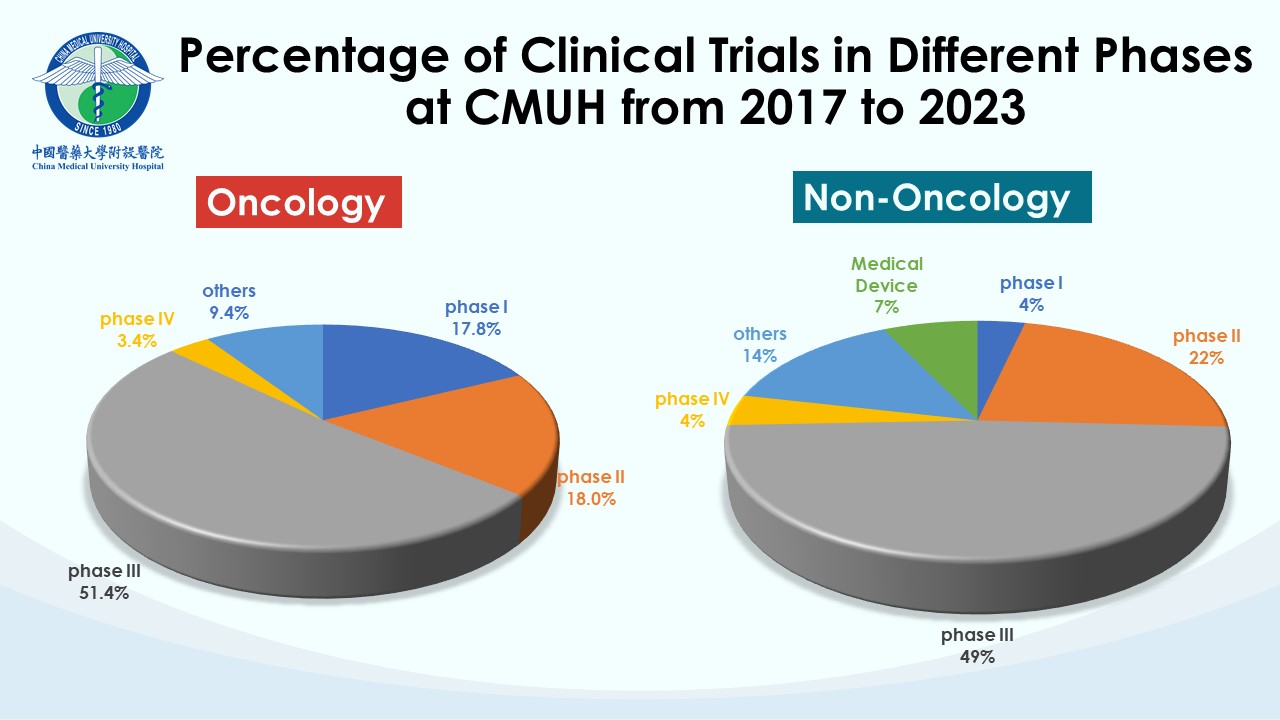
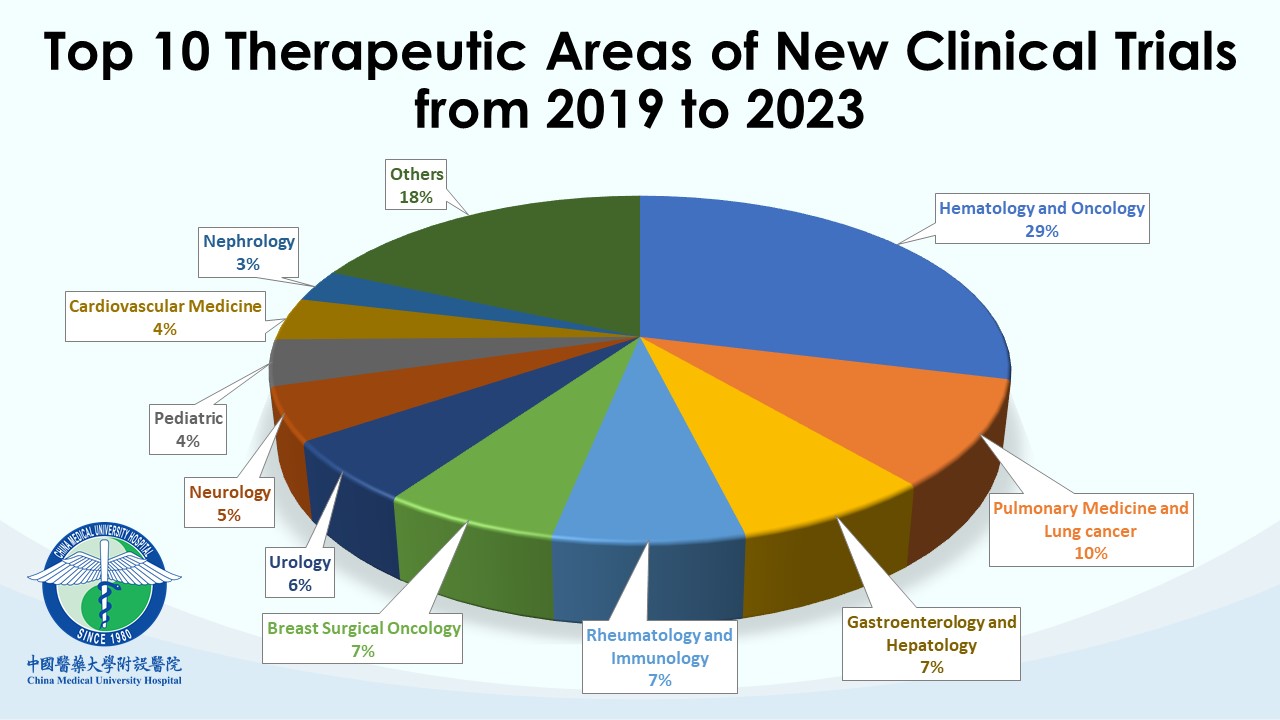
Each team within the CTC undertakes distinct professional responsibilities to advance, supervise, and manage clinical trial affairs in the hospital. CMUH has been a trusted collaborative partner for many global pharmaceutical companies and contract research organizations (CROs). Our clinical trial partners include the world’s top 50 international pharmaceutical companies. So far, CMUH-CTC has contracted with more than 420 clinical research companies and has conducted over 1,600 clinical trials with a wide scope of therapeutic trials, including over 110 phase I studies. CMUH will enhance cooperation with the biopharmaceutical industry in clinical trials by leveraging the long-term partnership between CTC and domestic and international companies to improve the efficiency of subject recruiting and clinical trial research capacity. Our goal is to speed up the smooth launch of new drugs and medical devices and promote the health and well-being of the whole people.